En nuestro grupo trabajamos en el desarrollo de nuevos métodos de síntesis de complejos organometálicos, fundamentalmente de elementos de los primeros grupos de las series de transición. Nuestro objetivo es modular las propiedades de los centros metálicos a través de la correcta elección de los ligandos.
Los complejos organometálicos encuentran aplicación en áreas tan diversas como la catálisis, la química bioinorgánica, la síntesis orgánica o la modelización de sistemas complejos, entre otras muchas.
Publicaciones científicas recientes:
Novel Titanocene Y derivative with albumin affinity exhibits improved anticancer activity against platinum resistant cells
Journal of Inorganic Biochemistry (2024) https://doi.org/10.1016/j.jinorgbio.2024.112520
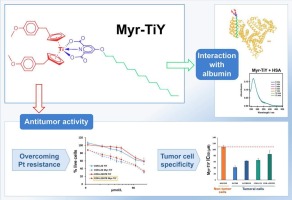
In this study we describe a new titanium complex containing a titanocene Y fragment, a tridentate ligand, and a long aliphatic chain that facilitates a non-covalent interaction with albumin. This compound shows tumor cell specificity and improved antitumor activity against platinum resistant human cancer cell lines.
New titanocene derivative with improved stability and binding ability to albumin exhibits high anticancer activity
Journal of Inorganic Biochemistry (2021) https://doi.org/10.1016/j.jinorgbio.2021.111562
In this study we describe a new titanium complex containing a titanocene fragment, a tridentate ligand, and a long aliphatic chain to facilitate a non-covalent interaction with albumin. UV–Vis absorption and fluorescence spectroscopy show high stability and strong interaction with human serum albumin. This compound shows antitumor activity in vitro in human cancer cell lines.
Organometallics, 2018, DOI: 10.1021/acs.organomet.8b00209
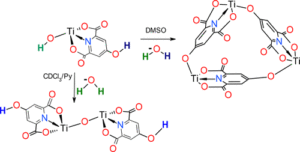
In this study we compare the properties of bis-cyclopentadienyl and monocyclopentadienyl titanium chelidamate complexes with the behavior of previously reported titanium dipicolinate derivatives. We have seen that the OH moiety in the chelidamate ligand does not increase the solubility of the titanocene complex in neutral D2O but allows its solubilization in basic media. In the case of monocyclopentadienyl complexes we have been able to isolate a titanium hydroxide compound that evolves in DMSO solution to render a neutral trimetallic cyclic architecture in which the chelidamate ligand behaves as an organic linker. Moreover, in the presence of a base, in CDCl3, that compound reacts to yield a dimetallic oxide. The hydroxide compound also can be deprotonated with NaOH to render a water soluble Ti-Na heterometallic complex that crystallize as a linear polymer. The theoretical calculations reveal that the two hydroxyl groups of that compound show significant differences on their nucleophilic and electrophilic character that can justify its reactivity in polar media.

